What is Electrochemical impedance spectroscopy?
Electrochemical Impedance Spectroscopy (EIS) is a powerful, non-destructive electrochemical technique used to characterize the electrical properties of materials and interfaces.
View instruments with EIS functionality
The fundamental principle of EIS involves applying a small amplitude, sinusoidal pertubation (AC) signal (e.g., current, potential) across an electrochemical system and measuring the resulting AC signal of interest.
In the classical electrochemical techniques, where DC signals are used in techniques such as Cyclic voltammetry and Chrono methods, the current and potential measurements are presented in the time domain (i.e., as a function of time). In the case of EIS, by varying the frequency of the applied AC signal, the AC current and AC potential measurements are presented in the frequency domain (i.e., the impedance of the electrochemical system is measured as a function of frequency) providing insights into the various and multiple sequential or overlapping processes occurring at the electrode-electrolyte interface, such as charge transfer, diffusion, double layer charging, adsorption and many others.
What are the benefits of the EIS technique?
The development of the electronic and digital potentiostats and advanced software features for EIS data modeling and analysis has revolutionized this technique, making it indispensable in electrochemical research and testing.
The primary benefit of EIS lies in its ability to separate and quantify individual parts which make up the complex electrochemical processes, creating incredibly information-rich results. Additionally, EIS is non-invasive, not destructive, and highly sensitivity to subtle changes in material properties or surface phenomena.
Where is EIS used?
EIS is a highly versatile and popular electrochemical technique, extensively used for characterizing:
- Interfacial processes: For mechanistic studies, adsorption, and electrosorption
- Reaction kinetics, mass transport, and diffusion processes
- Solution resistance, charge transfer resistance, and double-layer capacitance measurements
- Geometrical effects (linear, spherical, cylindrical) on mass transfer
EIS is used across diverse application fields to provide crucial information for material development, performance testing, optimization, and failure analysis, including:
- Corrosion Science: Determining corrosion rates, understanding material degradation, and studying reaction mechanisms.
- Battery Research: Evaluating state-of-health (SOH), internal resistance, and predicting battery lifetime for lithium-ion batteries, solid-state batteries, and more.
- Fuel Cells: Optimizing catalyst performance and membrane efficiency.
- (Bio)sensors: Characterizing sensitivity, response time, and selectivity.
- Coatings and Paints: Assessing the quality and protective properties of coatings.
- Conductive Polymers: Evaluating ionic and electronic conductivity, doping, degradation, swelling, and water uptake.
- Self-Assembled Monolayers: Studying molecular orientation, packing density, defects, electron transfer, and binding mechanisms.
- Semiconductors: Analyzing charge transport recombination, carrier dynamics, and the impact of doping and defects.
What should you consider when choosing your EIS instrument?
Selecting a suitable EIS system is crucial for successful experiments. Here are the most important factors to consider:
- Frequency Range: How fast (high frequencies) or slow (low frequencies) are your processes of interest?
- AC Amplitude and DC Value: Consider the maximum and minimum AC amplitude and DC values for applied and measured signals (current and potential), based on your samples and experimental requirements.
- Measurable Impedance: Consider the maximum and minimum measurable impedance at given frequencies, based on the samples and experimental requirements
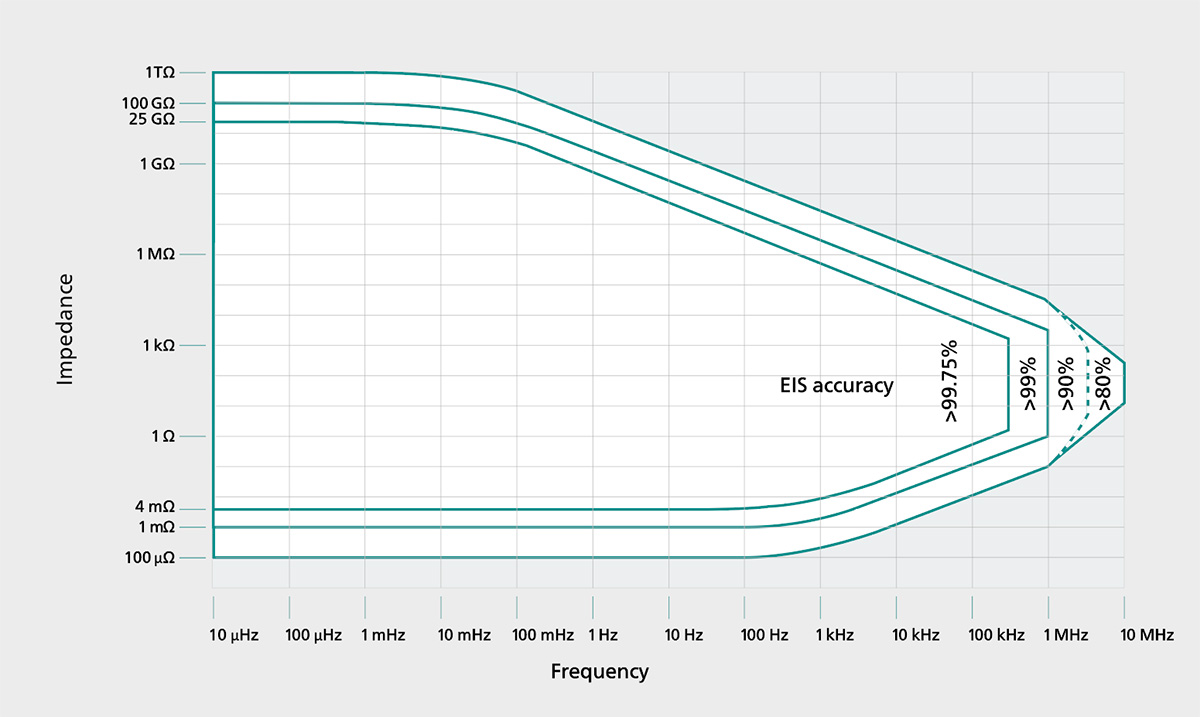
- Accuracy (EIS Contour Plot): Evaluate the specified accuracy of the EIS measurements across your frequency range of interest, i.e. the EIS contour plot
- EIS measurement on two electrodes simultaneously: Can the instrument measure EIS simultaneously on two electrodes (e.g., both the anode and cathode of a battery)?
- Software for Instrument Control: Look for user-friendly software with default procedures, as well as flexibility for routine and exploratory work.
- Data Analysis Software: Ensure robust software for EIS modeling and interpretation is available.
- Integration with Accessories: Check compatibility with accessories such as temperature control, various cells, holders, and liquid handling systems.
- Application and Technical Support: Is reliable local application and technical support available?
Why Choose Metrohm Autolab for EIS?
- Widest EIS frequency range on the market: 10 µHz to 10 MHz, capturing both very slow and incredibly fast electrochemical processes
- Highest EIS Accuracy: up to 99.75%
- Maximum AC amplitude: Measure any sample type with a maximum AC amplitude of 6 A or 10 V
- Simultaneous EIS on 2 electrodes (e.g., anode and cathode)
- External EIS capabilities at high current densities: Able to measure external EIS by modulating light intensity (IMVS/IMPS), electrode rotation (hydrodynamic EIS), or controlling external loads/power supplies
- Software with default procedures: Access a wide range of default procedures for potentiostatic, galvanostatic, time scan, frequency scan, and potential scan EIS
- Fit & Sim with circuit library: Streamline EIS data analysis with a user-friendly Fit and Simulation tool that includes a built-in circuit element library, allowing users to easily build and test equivalent circuit models
- Computer-Free Operation: Enhance efficiency and data safety during long measurements with onboard memory for up to 10 Million data points in our potentiostats, allowing computer-free operation
- Dedicated Local Support: we have local experts for professional application and technical support in over 80 countries
EIS CONTOUR PLOT - VIONIC
Autolab Instruments with EIS functionality

VIONIC powered by INTELLO
- Single channel, all-in-one potentiostat/galvanostat
- Maximum EIS frequency: 10 MHz
- Simultaneous EIS on 2 electrodes
- Maximum AC amplitude (p-p): ± 6 A / ± 10 V
- Maximum current: ± 6 A
- Dual-mode compliance voltage: ± 10 V / ± 50 V
- Dynamic current ranging: 1 nA - 6 A

Autolab Multichannel line: M101/M204
- Multichannel potentiostat/galvanostats
- Maximum EIS frequency: 1 MHz
- Maximum AC amplitude (M204, p-p): ± 0.2 A / ± 1 V (± 10 A with Booster / ± 10 V external EIS)
- External EIS functionality
- Up to 6 channels with EIS in one cabinet
- Maximum current: ± 400 mA (M204) and ± 100 mA (M101)
- Maximum current up to ± 10 A with Booster10A (M204)

Autolab Modular line: AUT302N
- Single channel modular potentiostat/galvanostat
- Maximum EIS frequency: 1 MHz or 10 MHz with optional ECI10M
- Maximum AC amplitude (p-p): ): ± 1 A / ± 1 V (± 20 A with Booster / ± 10 V external EIS)
- External EIS functionality
- Maximum current: ± 2 A or ± 20 A with optional Booster20A
- Compliance voltage: ± 30 V
- Upgradeable with up to 11 optional modules

Autolab compact line: AUT204 and Autolab IMP
- Single channel compact potentiostat/ galvanostat
- Maximum EIS frequency: 1 MHz
- Maximum AC amplitude (AUT204, p-p): ± 0.2 A / ± 1 V (± 10 A with Booster / ± 10 V external EIS)
- External EIS functionality
- Maximum current: ± 400 mA (AUT204) and ± 100 mA (Autolab IMP)
- Maximum current up to ± 10 A with Booster10A (AUT204)
- Compliance voltage: ± 20 V (AUT204) and ± 10 V (Autolab IMP)
Contact our local experts for tailored recommendations based on your EIS applications.
Other technical resources about EIS
Application notes
List of application notes related to EIS
FAQ - Electrochemical impedance spectroscopy (EIS)
Analyzing Electrochemical Impedance Spectroscopy (EIS) data is essential for understanding key electrochemical processes such as charge transfer, ion diffusion, and electrode surface behavior. The measured EIS data are usually represented in Nyquist plots and Bode plots. The most common approach to EIS data analysis involves a combination of visual interpretation of these plots and equivalent circuit modeling. In this way, the experimental EIS data are fitted to a theoretical electrical equivalent circuit that best describes the electrochemical system under study.
The typical equivalent electrical components (equivalent elements) include: resistors (R), capacitors (C), inductors (L), constant phase elements (CPE or Q), Warburg impedance (W), among others.
Together with validation techniques like Kramers-Kronig transforms, EIS data can be effectively analyzed to gain valuable insights into the behavior of electrochemical systems. Having a robust but user friendly, flexible fitting tool is important for a accurate and efficient EIS data analysis.
Learn more about our potentiostat and galvanostat solutions.
We offer an industry-leading 3-year warranty for all our instruments, along with local support available in over 120 countries worldwide. This ensures a rapid response for sales and service, usually within 48 hours.